-
About
- Kyoto Prize
-
Research Grants
-
News
This website uses cookies to improve the user experience. If you continue on this website, you will provide your consent to our use of cookies.
About
Research Grants
News

InaRIS Fellow (2021-2030)
Professor,Institute of Low Temperature Science, Hokkaido University*Profile is at the time of the award.
2021InaRISBiology & Life sciences
I am happy to have the long-term support of InaRIS for 10 years, which will allow me to stably conduct research on hibernation, a phenomenon that occurs on a yearly basis, and to have time to deeply consider unexpected experimental results. It will also be of great help that I can have the support both for and from the researchers and technicians who work with me. I will challenge myself to uncover the mysteries of hibernation.
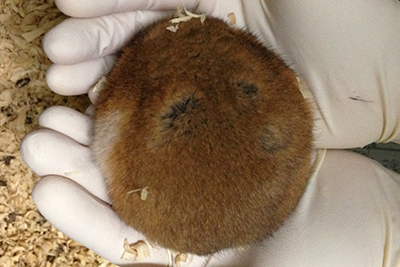
To elucidate molecular mechanisms of mammalian hibernation, we have studied several aspects of hibernation in Syrian hamsters as a model mammalian hibernator. We found that hibernation consists of not only an energy-saving program but also of an intrinsic program that converts the circadian rhythm of body temperature and adapts it to the post-hibernation season. We also identified marker genes for seasonal body remodeling and genes responsible for cold resistance in this species.
I have attempted to elucidate the molecular mechanisms of the “three major mysteries of hibernation” related to mammalian hibernation, which is a flexible adaptation to the winter environment, using the Syrian hamster (hereafter referred to as “hamster”) as a model mammalian hibernator.
Understanding these molecular mechanisms is more than just the basic biological value of curiosity-driven exploration. The development of methods to partially induce these molecular mechanisms in non-hibernator humans, which are usually considered to be in a “non-tolerant” state of hibernation, could lead to the long-term preservation of transplanted organs, reduction of damage in the event of stroke or heart failure, prevention of lifestyle-related diseases and sarcopenia, and prevention and treatment of seasonal affective disorders in humans, which are hypothesized to be vestiges of hibernation. The following describes the progress made since the adoption of InaRIS in FY2021.
(1) Molecular mechanisms of cold resistance in mammalian hibernators: Mammalian cells that cannot hibernate(non-hibernators), such as humans and mice, die within one to several days of being cultured at low temperatures. This cold-induced cell death in non-hibernators is known to resemble ferroptosis, a form of cell death in which certain cancer cells die via iron ion-dependent lipid peroxidation, induced by anticancer drugs. In contrast, mammalian hibernators’ cells, including hamsters, do not exhibit cold-induced ferroptosis even when cultured at low temperatures. Although this phenomenon has been known for a long time, the molecules and genes involved in cold resistance of mammalian hibernators remain unknown. We found that the cold resistance of hamster hepatocytes was diet dependent. Subsequent analyses revealed that hamsters contain high concentrations of vitamin E in the liver and blood to prevent lipid peroxidation and cold-induced ferroptosis (Anegawa et al., Commun Biol, 2021). Further analysis of why hamsters retain high concentrations of vitamin E is currently underway. However, we found that vitamin E alone could not explain the cold resistance of the hamsters. Cancer cell lines derived from hamsters are tolerant to low temperatures, even when cultured in a medium that does not contain vitamin E. Therefore, we conducted gain-of-function and loss-of-function screening for cold resistance using hamster cancer cell lines that show low-temperature tolerance and human cancer cell lines that do not show cold resistance. To date, we have identified the GPx4 gene as one that confers cold resistance to human cancer cells through overexpression and have successfully identified several candidate genes whose loss of function causes loss of cold resistance in hamster cells.
(2) Molecular mechanisms of seasonal body transformation in preparation for hibernation: Hamsters undergo hibernation by remodeling their entire body into a winter-specific body when kept under warm long-day conditions, but exposed to cold short-day conditions for a long period of time. We succeeded in obtaining gene expression profiles of various organs whose functions are important during hibernation, such as white fat, liver, skeletal muscle, pituitary gland, heart, and brown fat. In the liver, we focused on Season-Dependent Genes (SDGs), a group of genes with unknown functions that show more than 100,000-fold expression changes between summer and winter specifications, and constructed an adeno-associated viral system to manipulate their functions. We are also constructing an experimental system for the functional verification of other organs by narrowing down the genes to be focused on. Among the many genes whose activity is greatly reduced during hibernation in the pituitary gland and skeletal muscle, we analyzed a group of genes that are expected to be involved in systemic physiology and physiological changes in the skeletal muscle. However, new results were obtained from completely different perspectives. How circadian and behavioral rhythms change during hibernation is still a matter of controversy. We applied a data logger, which has made great progress in recent years, to obtain the body temperature and activity levels of animals for a long period of time over a year. From the analysis, we found that the body temperature rhythm disappears during prolonged hibernation, and that the circadian and activity rhythms of the body temperature also return to the summer long-day type during autonomous recovery from the prolonged hibernation period (Nakagawa and Yamaguchi, Proc B,2023). Hibernators survive winter in the absence of daylength signals by holing up in their burrows with short-day rhythms when day length is shorter, but when they emerge from their burrows in early spring, they are suddenly exposed to longer daylengths. Therefore, the autonomous return of body temperature and activity rhythms to the summer-specific long-day type is advantageous for environmental adaptation in early spring. Thus, hibernation is not only an energy-saving program to conserve energy and survive the winter season when food is scarce but also an autonomous recovery program to adapt the body rhythm to the coming spring. This is an example of the flexibility and resilience of organisms under the theme of this study.
(3) Molecular mechanisms for the torpor-arousal cycle: Our understanding of the genes, molecules, and neural circuits required to induce drastic temperature fluctuations during hibernation is lagging. Several studies have reported that the activation of specific neurons by pharmacological methods or optogenetics can induce lowered body temperature similar to hibernation, but there are no reports on the genes required for hibernation activation itself. We have succeeded in identifying genes that delay or inhibit the onset of hibernation by creating genetically engineered hamsters and are now analyzing the mechanism of action of these genes. A comparative genomic approach between hibernators and non-hibernators is also useful in answering this question. We have just begun analyzing this point as well.
Spontaneous recurrence of a summer-like diel rhythm in the body temperature of the Syrian hamster after hibernation. Nakagawa S, Yamaguchi Y*. Proceedings of the Royal Society B: Biological Sciences 290, 2009, 2023. doi.org/10.1098/rspb.2023.0922
Step-by-step protocols for non-viral derivation of transgene-free induced pluripotent stem cells from somatic fibroblasts of multiple mammalian species. Yoshimatsu S, Yamazaki A, Edamura K, Koushige Y, Shibuya H, Qian E, Sato T, Okahara J, Kishi N, Noce T, Yamaguchi Y, Okano H*. Development Growth & Differentiation 64(6):325-341, 2022. https://doi.org/10.1111/dgd.12798
Generation of transgenic mice expressing a FRET biosensor, SMART, that responds to necroptosis. Murai S, Takakura K, Sumiyama K, Moriwaki K, Terai K, Komazawa-Sakon S, Seki T, Yamaguchi Y, Mikami T, Araki K, Ohmuraya M, Matsuda M, Nakano H*. Communcations Biology 5(1):1331, 2021. https://doi.org/10.1038/s42003-022-04300-0
Hepatic resistance to cold ferroptosis in a mammalian hibernator Syrian hamster depends on effective storage of diet-derived α-tocopherol. Anegawa D, Sugiura Y, Matsuoka Y, Sone M, Shichiri M, Otsuka R, Ishida N, Yamada KI, Suematsu M, Miura M, Yamaguchi Y*. Communications Biology 4(1):796, 2021. https://doi.org/10.1038/s42003-021-02297-6
Evidence for the involvement of caspases in establishing proper cerebrospinal fluid hydrodynamics. Yoshida A, Kawata D, Shinotsuka N, Yoshida M, Yamaguchi Y*, Miura M. Neuroscience Research 170:145-153, 2021. https://doi.org/10.1016/j.neures.2020.12.006
Hibernation is a phenomenon that achieves the “flexibility” and “robustness” of life. Hibernation in mammals is a outlive strategy to survive severe conditions such as low temperature and starvation through inducing systemic suppression of metabolism and hypothermia. Most mammals, including humans, are unable to hibernate, but some mammals, such as bears and squirrels, are able to. Unlike non-hibernators, hibernators are resistant to tissue damages caused by prolonged hypothermia and those during the recovery process from the low body temperature. Hibernators are also known to be resistant to disuse muscle atrophy caused by immobility associated with hibernation. Furthermore, hibernators have many fascinating characteristics associated with hibernation, such as seasonal fluctuations in appetite and body weight, efficient burning of stored fat, endogenous seasonal timers.
However, mechanisms for the regulation of these physiological changes and for the characteristics associated with hibernation are still largely unknown, possibly due to a number of technical limitations. Hibernation is the remaining frontier of life science in the 21st century. This study not only deepens our knowledge and understanding of this interesting life phenomena, but is also expected for medical and pharmaceutical applications, such as artificial hibernation and surgery under hypothermia.
Dr. Yamaguchi’s research proposal addresses these issues head-on. Over the past decade, he has performed experiments using the Syrian hamster, which is relatively easy to induce hibernation in the laboratory. With introducing recently developed technologies, he has identified genes involved in hibernation and verified their functions in animals. To date, he has found a drastic changes in white adipose tissues precedes the onset of hibernation in Syrian hamsters acclimated to winter-like condition, and suggested production of endogenous ligands that enhance the ability of lipid catabolism and anabolism simultaneously in the winter-like body of the animals. He has also discovered dietary nutritional factors which confer cold tolerance. He is now identifying candidate genes that affect cycles of the deep torpor bout and periodic arousal.
Based on the above results obtained by his on-going research, this study aims to elucidate the molecular mechanisms of the three major mysteries of hibernation: cold tolerance, seasonal body changes, and hibernation triggers. Thus, his research proposal is full of original ideas and will open up the unexplored new world of life science, hibernation. Dr. Yamaguchi is a promising scientist for a new era of hibernation research. With the support of the InaRIS Fellowship, he is expected to realize his novel ideas and to unravel the mysteries of hibernation during the next 10 years.
The Inamori Foundation announced the 2025 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program on March 14, 2025.

The Inamori Foundation started to accept applications for the 2025 Inamori Research Institute for Science (InaRIS) Fellowship on May 20, 2024.

On April 20, the 2024 InaRIS Fellowship Award Ceremony was held at a hotel in Kyoto.

The Inamori Foundation announced the 2024 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program on March 15, 2024. This year, we will welcome two new fellows, Hiroshi Suzuki (Professor, Graduate School of Medicine, Nagoya University) and Ayuko Hoshino (Professor, Research Center for Advanced Science and Technology, The University of Tokyo), who were...

The Inamori Foundation started to accept applications for the 2023 Inamori Research Institute for Science (InaRIS) Fellowship on May 23, 2022.

From the left: Executive Managing Director Shoichi Himono, InaRIS Chair Shigetada Nakanishi, the 2023 Fellows Yoshihiro Tanaka and Yasutaka Kamei, and Chair of Selection Committee Hiroto Yasuura On April 22, the InaRIS Fellowship Award Ceremony was held at a hotel in Kyoto. The 2023 InaRIS Fellows were recruited under the theme “Transcending the Future with...
The Inamori Foundation announced the 2023 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program on March 17, 2023.

The Advisory Board Meeting of the Inamori Research Institute for Science (InaRIS) Fellowship Program was held at the Inamori Foundation in Kyoto, on October 4. There the committee members and the fellows had a discussion over their research themes. The InaRIS Fellowship Program was established in 2019 to encourage researchers to pursue their curiosity...

On April 23, InaRIS Fellowship Award Ceremony was held at a hotel in Kyoto for the first time since the establishment of the Fellowship in 2019.

The Inamori Foundation announced the 2022 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program on March 18, 2022.

The Advisory Board Meeting of Inamori Research Institute for Science (InaRIS) Fellowship Program was held online on October 3 for the Committee members and the fellows to have a discussion over their research themes.

The Inamori Foundation started to accept applications for the 2022 Inamori Research Institute for Science (InaRIS) Fellowship on May 21, 2021.
On March 19, 2021, the Inamori Foundation announced the two 2021 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program, Hiroshi Nishimasu (Professor, Research Center for Advanced Science and Technology, The University of Tokyo) and Yoshifumi Yamaguchi (Professor, Institute of Low Temperature Science, Hokkaido University) were selected from 66 applicants. InaRIS Fellowship Program...

Inamori Research Institute for Science (InaRIS) Fellowship Program is a research grant program that allows researchers to pursue challenging projects over a 10-year period. The Advisory Board Meeting of InaRIS Fellowship Program was held on October 26 at the Inamori Foundation in Kyoto to have a discussion over the fellows’ research themes. InaRIS Fellowship Program...

The Inamori Foundation started to accept applications for the 2021 Inamori Research Grants and Inamori Research Institute for Science (InaRIS) Fellowship on May 21. The Inamori Research Grants Program was established in 1985 to foster diverse and creative research activities by providing opportunities to challenge many potential themes. This program has little scrutiny on how...
On April 10, 2020, the Inamori Foundation announced the 2020 fellows for the Inamori Research Institute for Science (InaRIS) Fellowship Program. As the first two InaRIS fellows for this new program, Tadashi Takayanagi (Professor, Yukawa Institute for Theoretical Physics, Kyoto University) and Atsushi Noguchi (Associate Professor, Graduate School of Art and Sciences, The University of...

The InaRIS Fellowship Program grants 10 million yen per year over 10 years (totaling 100 million yen) to researchers engaged in investigations into topics with high potential based on a grand vision, providing them with opportunities to indulge themselves in research activities and pursue the possibility of triggering a quantum leap in science. The keyword...

The Inamori Foundation starts to accept registration for the Inamori Grants and Inamori Research Institute for Science (InaRIS) Fellowship.

Today, the Inamori Foundation announced an establishment of the new grants program: “Inamori Research Institute for Science (InaRIS) Fellowship Program” in the press conference at Ministry of Education, Culture, Sports, Science and Technology.
Biology & Life sciences